
Introduction: Fostamatinib is an oral, potent inhibitor of spleen tyrosine kinase (SYK) with proven efficacy and a manageable safety profile for the treatment of ITP. SYK is situated in an intracellular signaling pathway upstream of Bruton's tyrosine kinase (BTK). Long-term safety data on fostamatinib at various dosing regimens (up to 150 mg BID) has been collected in >4000 patients with ITP, rheumatoid arthritis (RA), and other autoimmune, allergic and neoplastic disorders. The safety and tolerability of fostamatinib were consistent across different patient populations (apart from disease specific events). We present a summary analysis of the fostamatinib safety data from the ITP and RA studies.
Methods: Fostamatinib safety data from 2 randomized, double-blind, placebo-controlled, phase 3 studies and the long-term, open-label, extension (OLE) study in ITP were pooled and are based on a starting dose of 200 mg/day, which was increased to 300 mg/day after 4 weeks in 88% of patients. Fostamatinib safety data from 13 phase 2/3 studies in RA were pooled and are based on a dosing regimen of 100-150 mg/day (n=1232) or 200-300 mg/day (n=2205).
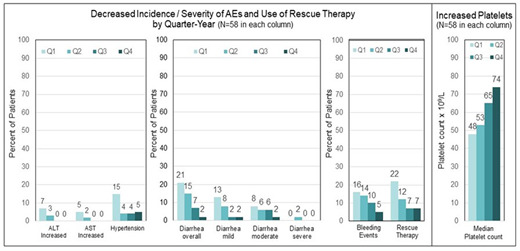
Results: The pooled data set for ITP included 146 patients; 60% were female, and the median age was 53 years (range 20-88). The mean duration of fostamatinib treatment was 19 months (range <1-62 months), representing 229 patient exposure years. Adverse events (AEs) were reported in 87% of patients, and 63% were mild to moderate. Serious AEs were reported in 31% of patients. The incidence of diarrhea, hypertension, alanine aminotransferase increase (ALT), and aspartate aminotransferase (AST) increase was evaluated in 58 patients who received fostamatinib for ≥1 year. This enabled a comparison of the incidence of these AEs in quartiles over the first year to assess the cumulative effects of fostamatinib. The AEs were reported with decreasing frequency during the second, third, and fourth quarters of fostamatinib treatment compared with the first quarter of the initial year of treatment in the 58 patients (see Figure 1). In the same 58 patients, the use of rescue therapy decreased while median platelet counts increased each quarter of the first year.
The pooled data set for RA included 3437 patients who received fostamatinib; 83% were female, and the median age was 54 (range 18 -87). The mean duration of treatment was 18 months (range <1-81) representing 5134 patient exposure years. AEs were reported in 86% of RA patients and were mild to moderate in 73% of RA patients. Serious AEs occurred in 14%. In the placebo-controlled RA studies, 2414 patients received fostamatinib with 823 patient exposure years and 1169 received placebo with 367 patient exposure years. Despite a two-fold (125%) increase in exposure with fostamatinib vs placebo (823 vs 367 patient exposure years), there was only a 26% increase in AEs with fostamatinib vs placebo (68% vs 54%).
The most common events in the ITP and RA studies were diarrhea (36% and 24%), hypertension (22% and 19%) and nausea (19% and 8%), apart from disease-related AEs. Epistaxis (19% and 0.5%), petechiae (15% and 0.3%), contusion (12% and 2%), and fatigue (10% and 2%) are associated with ITP and were uncommon in the RA population. Rheumatoid arthritis was reported as an AE in 9% of patients with RA and in none with ITP. Some AEs may be dose-related, and one-third of the RA patients were on lower dosages (100-150 mg/day) than were generally given in the ITP trials (200-300 mg/day).
Conclusions: Fostamatinib has been evaluated in >4000 patients across different disease populations. Fostamatinib has a consistent and manageable safety profile. No new safety signals and no cumulative toxicity were observed with up to 81 months (6.8 years) of continuous treatment.
Tong:Rigel: Current Employment, Current equity holder in publicly-traded company. Numerof:Rigel: Current Employment, Current equity holder in publicly-traded company. Datangel:Rigel: Current Employment, Current equity holder in publicly-traded company. Masuda:Rigel: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal